Chemical Bonding
Chemical compounds are formed by the joining of two or more atoms.
A stable compound occurs when the total energy of the combination has lower
energy than the separated atoms. The bound state implies a net attractive force
between the atoms which causes a chemical bond. Two examples of chemical bonds
are:
Covalent bond:
bond in which one or more pairs of electrons are shared by two atoms.
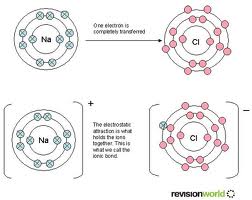
Ionic bond:
bond in which one or more electrons from one atom are removed and attached to
another atom, resulting in positive and negative ions which attract each other.

No comments:
Post a Comment